Gov. Mills issues statement on FDA’s emergency use authorization of Pfizer’s COVID-19 vaccine
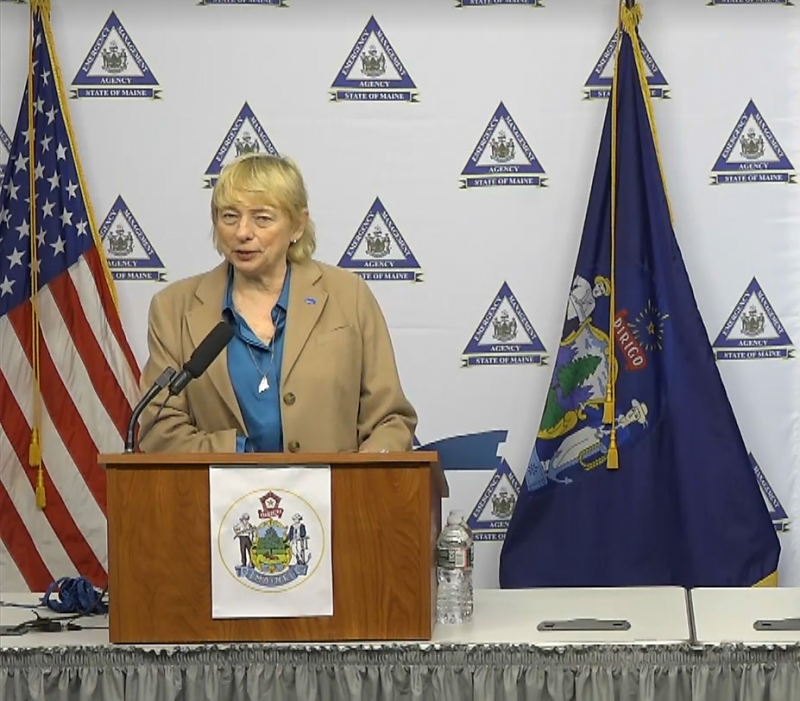
AUGUSTA — Governor Janet Mills released the following statement late Friday night in response to news the FDA has issued an emergency use authorization for Pfizer’s COVID-19 vaccine:
“The approval of Pfizer’s vaccine is a beacon of hope during this challenging time and further proof of the vast and remarkable power of science.
“Over the past several months, my Administration has worked closely with Maine hospital systems, health care officials, long term care facilities, pharmacies, and others to prepare to deliver the vaccine in the quickest, most efficient, and most equitable manner possible.
“With this approval, we are now on the verge of initiating this monumental effort, the largest mass vaccination initiative in our state’s history.
“As early as next week, we will begin vaccinating our frontline health care workers.
“While we will do this as quickly as we can, widespread vaccination will take much longer and be dependent on the supply of the Pfizer vaccine and any other vaccines that may gain approval in the coming weeks and months.
“It will take patience. In the meantime, Maine people must continue to take all the steps that we know will protect against the spread of the virus, like wearing a face covering, watching our distance, washing our hands, and avoiding gatherings whenever possible.
“But for tonight, let us extend our gratitude to the scientists who worked so hard to make this breakthrough possible.”
Event Date
Address
United States